Amagats
From the ideal gas law we can define the number of particles
at standard temperature (273.15 K) and standard pressure (1 atm =
1013.25 milli-bars). The ideal gas law can be written many ways, here
we will adopt the notation(s)
 Na k T/<mw> =
Na k T/<mw> =  Rg T
Rg Twhere, N is the number of molecules per unit volume, Boltzmann's constant k = 1.3806 · 10-16 erg/K, the gas constant
Rg = Na · k/<mw>, Avogadro's number, Na = 6.02 · 1023 particles per mole, <mw> is the molecular weight in grams/mole.
This number of molecules at STP is called Loschmidt's number and has the value,
The number of amagats of a gas is given as a ratio of the
actual number of particles at the given temperature and pressure to
Loschmidt's number. In planetary atmospheres the number density is a
function of height. We can calculate the thickness of an equivalent
atmospheric column at standard temperature and pressure. This is
denoted as cm-amagats or Km-amagats and is given by
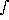 Ni(z) · dz
Ni(z) · dzHydrostatic equilibrium relates the pressure and vertical coordinates
 -
-  g dz, or dz = -dP/(
g dz, or dz = -dP/( g),
g), and the number density, Ni can be written as a function of the density,
 Na/<mw>, molecules/cm3
Na/<mw>, molecules/cm3where, qi is the volumetric fraction of species i.
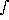 qi/<mw> · dP
qi/<mw> · dP
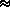 Na/(g N0) ·
( qi/<mw>) · P = H0 · P
Na/(g N0) ·
( qi/<mw>) · P = H0 · P
| Earth | Mars | Jupiter | Saturn | Titan | Uranus | Neptune | |
| g | 981.0 | 374.1 | 2425.3 | 1000.0 | 136.0 | 880.1 | 1110.5 cm/s |
| q(H2) | 1.00 | 1.00 | 0.90 | 0.96 | 1.00 | 0.85 | 0.85 |
| <mw> | 28.97 | 44.01 | 2.22 | 2.14 | 28.00 | 2.30 | 2.30 gm/mole |
| H0 | 7.88 | 13.61 | 37.45 | 100.50 | 58.85 | 94.08 | 74.56 Km-am/Bar |
| 1000/H0 | 126.9 | 73.49 | 26.70 | 9.95 | 16.99 | 10.63 | 13.41 mB/Km-am |
 PDS: The Planetary Atmospheres Node
PDS: The Planetary Atmospheres Node
